We Go The Extra Distance For Our Clients
Practice Areas
DePuy ASR Hip Implant Recall
Modern medicine is amazing because when some parts of our bodies start breaking down, they can easily be replaced with high-tech medical devices or medications. These medical interventions are typically extensively studied, carefully manufactured, and able to drastically improve someone’s quality of life. Unfortunately, modern medicine is not perfect, and it can sometimes make our bodies, and our lives, worse. This was certainly the case for the DePuy ASR Hip Resurfacing System and the ASR XL Acetabular System, both of which are defective metal-on-metal implants. These defective hip implants led to early failure and major health complications such as metallosis. Because of these problems, DePuy Orthopaedics Inc. issued a voluntary recall in 2010, which subsequently led to thousands of lawsuits.
Although DePuy’s parent company, Johnson & Johnson, settled these lawsuits with $4 billion, you can still take legal action, especially if you’ve directly suffered from the DePuy ASR hip implant recall. Below, our legal team breaks down everything you need to know about the DePuy hip implant recall and claims process.
If you have suffered physical, emotional, and financial damages from a defective hip implant, allow the Sandy Springs personal injury lawyers at Ashenden & Associates to fight for your justice. Call 770-394-8909 to schedule a free consultation at our law firm today.

What is a Hip Replacement?
A hip replacement is a type of surgery that involves removing the diseased or damaged portions of the hip joint and replacing them with a hip implant. Hip implants are typically made of hard plastic or metal, and they can significantly improve the mobility and quality of life of patients who suffer from severe pain and difficulty walking.
Common Reasons Why People Need Hip Replacement Surgery
The most common reasons why people need hip surgery include:
- Osteoarthritis is the most common type of arthritis. It’s often called “wear and tear” arthritis or degenerative joint disease because the soft tissue (cartilage) that protects joints erodes over time. Osteoarthritis can affect any overused joints, such as the knees. This condition is very common among older people who have led very active lifestyles or have had physically demanding jobs.
- Rheumatoid Arthritis is an autoimmune disease. This means that the body’s immune system attacks healthy cells and systems in the body, leading to inflammation and other symptoms. In the case of rheumatoid arthritis, the immune system attacks healthy joints, resulting in severe pain, inflammation, poor mobility, and possible disfigurement.
- Osteonecrosis basically means the death of bone tissue. This condition occurs when blood flow to a bone or joint is restricted. Without adequate blood flow, the affected bone or joint tissue will erode and collapse.
Hip surgery is a last resort option for people suffering from severe pain, inflammation, and difficulty walking. Before recommending a hip replacement, doctors will often suggest less invasive treatments such as non-steroidal anti-inflammatory drugs (NSAIDs), hot and cold therapy, physical therapy, and assistive devices such as canes or walkers. If these options don’t work, and the patient’s mobility and quality of life suffers greatly, then hip surgery is the next best option.
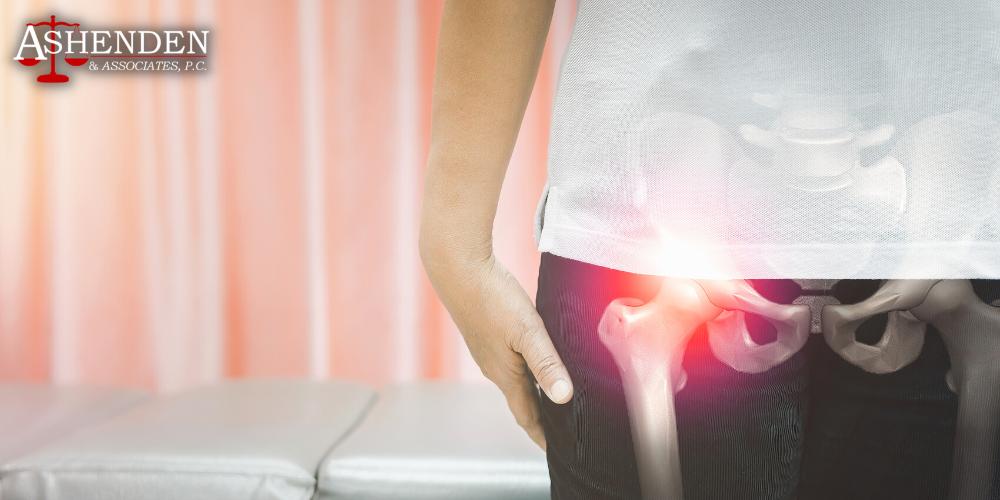
Common Types of Hip Implant Devices
There are many types of hip implants to choose from, including:
- Metal-on-Polyethylene: This type of implant is made up of a metal ball and a plastic (polyethylene) socket.
- Ceramic-on-Polyethylene: This type of implant is made up of a ceramic ball and a plastic socket.
- Ceramic-on-Ceramic: This type of implant is made up of a ceramic ball and socket.
- Ceramic-on-Metal: This type of implant is made up of a ceramic ball and a metal socket.
Metal-on-metal hip implants (metal ball and socket) used to be very common in the United States until patients began experiencing severe health complications and high failure rates, meaning many of the patients had to undergo revision surgeries.
If you’re a good candidate for a hip replacement surgery, your orthopedic doctor will help you decide on the best type of implant for your body, your lifestyle, and your activity levels.
What is the DePuy ASR Hip Implant?
The DePuy ASR system is a metal-on-metal hip implant device that is no longer available due to its high failure rates, health complications, and subsequent lawsuits.
DePuy Orthopaedics Inc. is a subsidiary of the large pharmaceutical company Johnson & Johnson. DePuy is among the largest hip implant manufacturers in the world. The ASR hip resurfacing system was among its most popular implant products before its recall, but the manufacturer offers a wide variety of other hip implant components and systems as well, including:
- Actis Total Hip System
- Bi-Mentum Dual Mobility Hip System
- Corail Hip System and KAR Systems
- Emphasys Hip Solutions
- Gripton TF Acetabular Revision System
- Pinnacle Hip Solutions
- Reclaim Modular Revision Hip System
- Summit Tapered Hip System
- Tri-Lock Bone Preservation Stem
Why Was the DePuy ASR Hip Implant Recalled?
The main reason why the DePuy ASR Hip Resurfacing System and the ASR XL Acetabular System were recalled in 2010 is because of high failure rates. In fact, data from the National Joint Registry showed that about 13% of patients (or one in eight patients) who got the ASR XL acetabular system required revision surgeries after only 5 years. The lifespan of the ASR Hip Resurfacing System wasn’t much better; the same data states that about 12% of patients also needed a revision surgery within 5 years. For reference, most hip implants are supposed to last about 15 years.
The DePuy hip implant was also recalled due to a health complication called metallosis. Metallosis, also known as metal poisoning, occurs when metal debris builds up in the body due to metal-on-metal prosthetic devices. This happens when the metal components rub together as the person moves and walks, which creates microscopic metal particles that are released into the body. Metal prosthetic devices are usually made of cobalt, chromium, titanium, nickel, and molybdenum. Even when these metal hip implants are removed and replaced, patients can still suffer from high cobalt and chromium ion levels as well as other signs of metallosis.
Metal ion levels in the blood can increase to dangerous levels and lead to symptoms such as:
- Psychological and neurological issues like depression, anxiety, brain fog, memory loss, and increased irritability
- Joint and/or groin pain
- Tinnitus and/or hearing loss
- Vertigo
- Skin rashes
- Difficulty breathing
- Heart issues like irregular heartbeat and heart failure
- Peripheral neuropathy
- Changes in vision
- Thyroid problems such as hypothyroidism or hyperthyroidism
- Poor kidney function
- Tissue damage surrounding the implant
- Swelling and pain around the hip implant
How Many People Were Affected By the DePuy Hip Implant Recall?
More than 93,000 people around the world received the ASR Hip Resurfacing System and the ASR XL Acetabular System by the time of the voluntary recall in 2010.
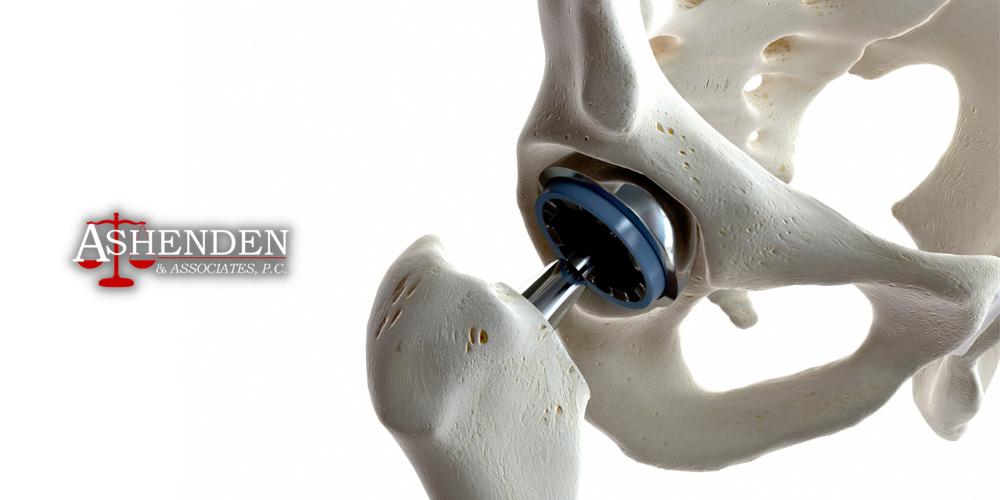
DePuy ASR Hip Implant Lawsuit and Settlement
At the time of the recall, Depuy Orthopaedics issued a statement saying it would cover any reasonable costs associated with revision surgery as well as the monitoring and treatment of other health complications, such as metallosis. Even still, patients implanted with the ASR Hip Resurfacing System and the ASR XL Acetabular System pursued legal action.
The plaintiffs claimed that DePuy created defective hip implants and that the company knew they were defective long before the recall was issued. Additionally, plaintiffs claimed that when DePuy Orthopaedics Inc. knew that ASR products were defective, they failed to take any sort of action, such as merely telling patients and orthopaedic surgeons about potential health complications.
Johnson & Johnson, the parent company of DePuy, settled thousands of lawsuits regarding the defective ASR system in 2013 for $4 billion.
Can I Still Sue for DePuy ASR Hip Implant Complications?
Yes, you can still file a lawsuit if you have suffered physical, emotional, and financial damages directly caused by the DePuy hip implant. Remember, metallosis symptoms can appear months or years after the metal implant is removed and replaced.
Additionally, a defective hip implant can cause permanent damage to the surrounding tissue. So even after you receive a better implant, you can still suffer from issues like poor mobility, pain, and joint inflammation. These issues can require frequent doctor’s appointments to manage, which can easily put the average middle class American in debt.
Our Sandy Springs defective medical device lawyers can review the details of your case, help you prove that DePuy Orthopaedics’ negligence caused your damages, and walk you through the entire claims process.
Damages for Defective Hip Implants
Our Sandy Springs defective medical device lawyers can help you recover financial compensation for the following types of damages:
- Medical expenses (including expenses associated with revision surgery)
- Physical therapy expenses
- Expenses associated with necessary medical equipment like canes, walkers, and wheelchairs
- Lost wages
- Loss of earning capacity if the health complications caused by your defective implant impedes your ability to complete all your job duties
- Permanent disability
- Physical pain and suffering
- Emotional distress
- Mental health counseling costs
- Decreased quality of life
- Loss of consortium


